Introduction
Psoriasis is a chronic genetic disorder that is mediated by the immune system; it mainly involves pathological changes in the skin and joints. The main symptoms include itching, flaky and scaly skin, pain, swelling, bleeding and skin damage [1]. However, the clinical course is difficult to predict [2]. Psoriasis poses many complications including physical disfigurement, chronicity, and its associated disability and comorbidities. Studying the pathophysiology of psoriasis can help control this complicated disorder whose impact on patients is far beyond the skin. To date there have been various standard local therapeutic approaches being applied that can help control the condition for months to years, such as corticosteroids, methotrexate, cyclosporine, acitretin, and phototherapy. However, complete recovery from psoriasis through these treatments has not been reported. Therefore, researchers worldwide are mainly considering to utilize various nanotechnology therapies to completely eradicate this condition [3–8]. New medication delivery carriers, particularly nanocarriers, may overcome certain disadvantages of conventional delivery methods, such as dose minimization, frequency of administration, and dose-dependent side effects.
Pathophysiology
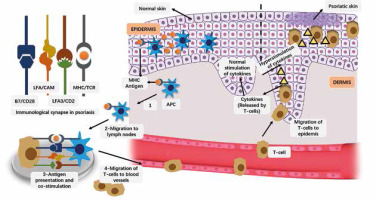
Psoriasis is an autoimmune disease without any obvious immunogen identified, but the basic pathophysiology of psoriasis is considered to be too much activation of parts of the adaptive immune system [4], more specifically, it is the collective result of a variety of cell types, including keratinocytes, dendritic and T cells, which are in a chronic inflammatory state due to the production of cytokines [9–13]. Other reasons of developing psoriasis include genetic heredity and mutations, weather, infections, stress, and skin lesions [14, 15]. Basic pathological events involved in psoriasis are presented in Figure 1.
Figure 1
Basic pathological events involved in psoriasis. The antigen attaches to the major histocompatibility complex (MHC) of antigen presenting cells (MHC). Antigen-bound APC migrates to lymph nodes. Antigens are presented to T cells and co-stimulation via APC. The activated T cell clones are redirected to blood vessels
Epidemiology
Psoriasis affects at least 125 million people worldwide, making it a major public heath challenge. The prevalence of psoriasis is equal among males and females [16], however it varies widely across different regions, ranging from as low as 0.5% (Asia) to as high as 8% in some European areas [16–18]. In the United States, approximately 3.2% of adults and 0.13% of children suffer from psoriasis [19–21].
The onset of psoriasis can be at any age, but is most common between the ages of 15 and 30 [22]. Environmental and genetic factors can influence the age of onset for psoriasis; for instance, the human leukocyte antigen (HLA)-C*06 allele has a strong correlation with early onset of psoriasis [23]. Besides, patient ethnicity, UV exposure, and the climate they live in are also considered to influence the prevalence of psoriasis, and recent studies have found a weak association between latitude and psoriasis prevalence [24].
Clinical diagnosis
Psoriasis is a typical skin disease that most dermatologists can easily identify based on its clinical manifestations, which are silver-white scales easily detaching from the skin, these scales usually are seen together with bright pink or red lesions with well-defined margins. The skin under the scales is pink, moist, and tender, and small bleeding spots will appear after the scales are scraped off from the moist skin. Normally, diagnosis would focus on investigation of any family history of psoriasis, and a clinical examination of the skin and nails, which includes an assessment of the distribution and morphology of psoriatic lesions. Sometimes, when psoriasis patients have atypical clinical presentations, dermatologists may also use skin biopsies or scrapes and blood analysis to eliminate other diseases and to verify the diagnosis. In addition, the Psoriasis Area and Severity Index (PASI) score is widely used to evaluate the severity of lesions in patients with psoriasis [25], and lattice system physician’s global assessment (LS-PGA) or psoriasis global assessment (PGA) have been used in routine clinical practice [26].
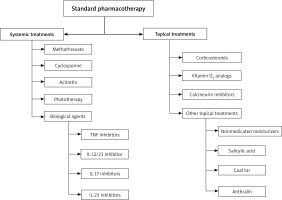
Standard pharmacotherapy
Therapeutic targets include improving skin, joint, and nail lesions, as well as improving the quality of life for patients. Although modern medicine has not discovered the complete cure for psoriasis, many treatment modalities can be utilized (Figure 2). In general, conventional treatment of psoriasis includes topical treatments, systemic treatments. The preferred treatment for patients with mild psoriasis with less than 10% of body surface area (BSA) is local topical treatment [27]. Topical treatments include corticosteroids, calcineurin inhibitors, vitamin D3 analogues, and other topical treatments (non-medicated moisturizers, coal tar, salicylic acid, and anthralin). Systemic treatments are commonly used for severe disease. The presence of comorbidities that are effectively treated and other patient-related factors should also be considered in the selection of systemic treatments.
Topical treatments
Corticosteroids
Using corticosteroids topically is the standard primary treatment for the majority of patients with localized or mild psoriasis; these corticosteroids have anti-proliferative, anti-inflammatory, and locally vasoconstrictive properties through downregulating pro-inflammatory cytokines coding genes. The therapeutic effect of topical corticosteroids varies from classification (established using vasoconstrictive assays), with class I being the most potent, and class VII being the weakest. For mild or localized psoriasis, there are multiple options regarding using topical corticosteroids. During the acute phase of active psoriasis, topical agents are used twice daily; afterwards, patients can choose a topical agent (vitamin D analogue, topical corticosteroid, or calcineurin inhibitor) twice a week when the lesions become quiescent, which can reduce the risk of recurrence [28]. Besides, the results of a meta-analysis showed that corticosteroid combined with vitamin D3 yielded the best outcomes for the scalp [29]. Other studies also found that combined formulations are more effective, producing less side effects and a recurrence is slower to return compared with monotherapy [30, 31]. However, long-term use can cause potential side effects, such as local skin alterations, hypothalamic-pituitary-adrenal axis suppression, and tachyphylaxis [32].
Vitamin D analogues
Another adjuvant for chronic psoriasis is vitamin D analogues. When applied topically, these analogues combine with vitamin D receptors on T cells and to those on keratinocytes to prevent the proliferation of keratinocytes and promote its differentiation. Topical agents include capotriol, calcitriol, etc. Among them, tacalcitol ointment is an efficient and harmless treatment for chronic plaque psoriasis that can be used for the long term, and systemic side effects at sensitive sites are minimal with overall satisfactory tolerability, but it is not as effective as capotriol [33]. Topical vitamin D agents have a modest effect when used on its own [34]. Vitamin D analogues are relatively safe and can be applied liberally on patients without any renal impairment. The major side effects include burning sensation and irritation seen in 35% of patients and usually diminish over time [35].
Calcineurin inhibitors
Inflammatory substance is considered to be an important factor in the pathogenesis of psoriatic skin lesions [36], and calcineurin inhibitors can inhibit the production of inflammatory substance. Tacrolimus ointment with difference strengths (0.03% and 0.1%) and pimecrolimus cream (1%) are the most commonly used topical calcineurin inhibitors that are commonly prescribed for the treatment of psoriasis in facial and intertriginous areas long-term without any adverse effect such as skin atrophy [37]. Clinically, it is recommended to increase permeability by blocking therapy or mixing these reagents with salicylic acid [38, 39]. Generally speaking, Tacrolimus and pimecrolimus are not thought to be teratogenic due to their low uptake systemically [40]. However, tacrolimus and pimecrolimus are present in breast milk, therefore not suggested for breastfeeding mothers.
Other topical treatments
Non-medicated moisturizers including salicylic acid, coal tar, and anthralin are less frequently used topical agents [41]. Moisturizers help to handle the pressure generated by the skin and the Koebner phenomenon [42]. Moisturizers can soothe dry skin, decrease desquamation, relieve itching, soften the skin cracks, and enhance the absorption of other topical drugs [43]. Moisturizers can be applied to pregnant women and children, but some patients may develop allergic symptoms due to sensitivity to moisturizing materials. Salicylic acid lowers the pH of the cuticle, thereby hydrates and softens the skin [44]. The combination of steroid hormone and salicylic acid can be used as first-line treatment for plaque psoriasis [45]. Side effects of salicylic acid include possible acute or chronic systemic poisoning, symptoms of the CNS, tinnitus, metabolic acidosis, and nausea and vomiting [46, 47]. Coal tar may inhibit DNA synthesis, thereby reducing keratinocyte overproliferation [48]. Side effects of using coal tar include bad odour, contact dermatitis, staining, erythema, folliculitis, tingling sensation, and corneal acanthoma formation; the use of PUVA and coal tar for treatment are not advocated to prevent the risk of skin cancer development [49, 50]. Anthralin may reduce keratinocyte proliferation through mitochondrial dysfunction to inhibit the activation of T cells and restore cell differentiation [51]. Furthermore, free radical production may enhance its action. Side effects of anthralin depend on the dose of treatment.
Systemic treatments
Methotrexate
Methotrexate is a folic acid analogue that stops the synthesis of DNA by blocking the production of purine and thymidine. Initially, the recommended dosage is 7.5–10 mg/week and increased to of 25 mg/week maximally [52]. Despite being widely used in clinical practice, there is a lack of substantial well-structured clinical research to evaluate the efficacy and safety of methotrexate. A placebo-controlled, randomized, double-blinded study found that nearly 40% of patients taking methotrexate improved 75% in the Psoriasis Area and Severity Index score, compared with only 18.9% improvement in 16 weeks for patients receiving placebo treatment [53]. The use of methotrexate for the treatment of psoriasis in children has not been approved [54]. The common side effects include feeling tired and nauseous, diarrhoea, and vomiting. A more serious side effect is hepatotoxicity [55].
Cyclosporine
Cyclosporine is an immunosuppressant of calcineurin inhibitor that suppresses T cells and is used for moderate to severe psoriatic patients [56]. It has also been shown to be effective in treating psoriatic arthritis [57–59]. Cyclosporine can rapidly relieve symptoms, but its many adverse effects and drug interactions restrict its long-term use. Hypertension, nephrotoxicity and non-melanoma skin cancer are obvious potential side effects.
Acitretin
Acitretin is an oral retinoid to treat moderate to severe psoriasis. As an adjuvant therapy to other systemic agents, acitretin has been established as an agent that improves efficacy, lowers dosage, and reduces the incidence of side effects [60–62]. However, acitretin is teratogenic, so women are advised not to plan any birth for three years after discontinuing the medication [63]. Mucocutaneous dryness, photosensitivity gastrointestinal troubles, and arthralgia are commonly seen side effects of acitretin; sometimes it can even induce elevated triglyceride levels and transaminitis.
Phototherapy
Phototherapy is often used for the treatment of moderate to severe psoriasis, particularly for psoriasis that is unresponsive to topical agents [64]. In addition, phototherapy can be combined with biologic agents for the treatment of severe psoriasis [65]. The main types of phototherapy used to treat psoriasis include psoralen plus UVA, broadband UVB, and narrowband UVB (NB-UVB). Generally, the frequently used first-line treatment is NB-UVB therapy because it is more effective and safer [66]. No evidence suggests that NB-UVB predisposes patients to skin malignancies [67]. Another treatment of psoriasis is targeted phototherapy, for instance excimer light. Although it is safe, the limited availability of phototherapy centres and the need for frequent treatments make this option very inconvenient for patients.
Biological agents
In recent years, the development and approval of biological agents for the treatment of moderate and severe psoriasis and psoriatic arthritis have received wide attention. There are four classes of biologic agents for the treatment of psoriasis: IL-12/23 inhibitor, TNF inhibitors, IL-23 inhibitors, and IL-17 inhibitors. Monoclonal antibodies used in the treatment of psoriasis and psoriatic arthritis that can achieve the inhibition of TNF include adalimumab (Humira) and infliximab (Remicade), and another TNF inhibitor used is etanercept (Enbrel). Ustekinumab is a drug that blocks interleukin 12 and 23 through inhibiting their shared p40 subunit, and it is also approved for treating both psoriasis and psoriatic arthritis. IL-17 inhibitors are a class of biological agents that target the IL-17 ligand or its receptor rapidly and generate powerful response and yield satisfactory sustainability in the treatment of plaque psoriasis patients. IL-23 inhibitors, including guselkumab, tildrakizumab and risankizumab (approved by the US FDA to treat adult plaque psoriasis), are a class of biological agents that precisely inhibit the p19 subunit of IL-23. Among biologic therapies, infliximab produces the fastest clinical response, with sustained response and improved quality of life in clinical trials. It seems that the biological effects of short-term treatment are more superior to these of traditional systemic medications [53], to date no previous evidence has suggested that biologics cause cumulative toxicity or drug interactions in long-term treatment.
Nanotechnology
Advantages of nanotechnology in treating psoriasis
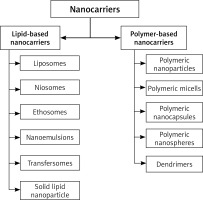
Nanotechnology could not only simplify diagnosis, but also make treatment more precise. In general, nanotechnology offers a better controlled, nontoxic, more secure and localized delivery of drugs than traditional treatments. A variety of nanotechnology colloidal carriers are popular for treating and controlling psoriasis because of their distinctive characteristics, such as liposomes, niosomes, transmitters, microspheres, micelles, dendrimers, glycosomes, solid lipid nanoparticles, etc. (Figure 3) [68–76].
Conventional topical treatments, such as ointments, creams, and gels have poor penetration and low absorption because of the barrier effect of the skin. Local administration of drug-loaded nanocarriers showed better permeability and efficacy at lower doses with minimum systemic side effects. As the particle size becomes smaller, the interaction with skin becomes better. Particles less than 20 nm can penetrate intact skin, however, diseased imperfect skin with a weaker barrier can allow larger particles to penetrate. Particles with the size between 50 and 100 nm are still on the stratum corneum layer [77, 78].
At present, these nanocarriers are becoming increasingly popular as delivery agents for anti-psoriasis drugs because of their non-toxicity, natural degradation, excellent biocompatibility and biodegradability; they do not cause any harmful inflammatory reaction and are easily excreted from the body. The performance of nanocarriers is stable, their porosity is adequate, their pore size distribution is ideal, and their interconnectivity, easy processability and malleability into desired shape, etc. [79–84] all contribute to their reputation.
Nanocarriers for psoriasis
Reports of nanocarrier delivery for psoriasis suggested that it improved efficacy and reduced toxicity compared to standard pharmacotherapy. To better elucidate the application of nanotechnology in the treatment of psoriasis, various nanocarrier-based formulations will be summarized [85, 86].
Lipid-based nanocarriers
Liposomes
Liposomes or lipid-based vesicles, which are produced by phospholipid, cholesterol and long chain fatty acids, are unilamellar or multilayer vesicles that can be seen microscopically [87, 88]. Drugs containing liposomes can be delivered in a variety of ways to treat psoriasis, such as IV injection, oral inhalation, topical application, and ocular delivery. The main advantages of the system include nontoxicity, non-immunogenicity, biocompatibility and biodegradability, increased stability, and ensuring that the encapsulated drugs are shielded from the external environment [89]. Sathe et al. examined a liposomic gel loaded with dithranol (particle size = 203.3 ±1.20 nm, PDI = 0.29 ±0.04 and zeta = -0.97 ±0.08) on an IMQ-treated mice model, and discovered an optimal anti-psoriatic property along with significant reductions in PASI and IL-17, IL-22, and IL-23 serum levels compared with controls [90]. Walunj et al. used a liposome-based gel containing cyclosporine (size = 111 ±1.62 nm, PDI 0.27 ±0.08, Z 41.12 ±3.56 mV) on an IMQ-treated murine model, and found a significant reduction in the severity of skin problems, along with IL-17, IL-22, and TNF-α [91]. Through the film hydration method, Gupta et al. developed liposomes, niosomes, and emulsomes containing capsaicin (CAP). Nanospheres allowed the maximum skin retention, especially in emulsified-gel formulations [92]. Srisuk et al. designed methotrexate (MTX) entrapped oleic acid-containing deformable liposomes, used for in vitro trans-epidermal delivery in psoriasis treatments, and the results suggested a promising use of liposomes to enhance the permeability of MTX in psoriasis treatment [93].
Niosomes
Niosomes (Nio) are non-ionic liposomes which constitute mainly of non-ionic surfactant, cholesterol, and other lipids [94]. They are available for parenteral, oral, and administration topically, and can also enhance the drug’s oral bioavailability and its penetration into skin [95]. Nio can be prepared as unilamellar or multilamellar vesicles in a similar way to liposomes [96, 97]. Nio also contain more surfactants than liposomes and may also provide a stronger penetrability [98]. Abdelbary et al. studied and discovered that MTX niosomes may have profound therapeutic application in topical delivery to treat psoriasis [99]. Abu Hashim et al. investigated the advantages of the Act nano niosomal gel as a topical drug delivery system, which includes significantly enhanced drug penetration and deposition into deeper skin layers, and reduced systemic uptake [100]. Meng et al. prepared Nio hydrogels containing Cel, which was effective in the treatment of IMQ-induced psoriasis-like mouse models. Encapsulation of Cel into Nio improved the water-solubility and permeation of Cel in the skin, and the anti-psoriasis activity was significantly improved in the mice model [101].
Ethosomes
Ethosomal carriers are constituted mainly of alcohol (ethanol & isopropyl alcohol at relatively high concentrations), phospholipids (phosphatidylserine, phosphatidylcholine, phosphatidic acid), and water; these carriers are able to catch hydrophilic, lipophilic, and amphiphilic drug molecules with varying characteristics [102, 103]. Ethosomes range from 30 nanometres to a few microns long [104]. Dubey et al. found that the enhanced accumulation of MTX in the skin through ethosomal carriers could help to improve targeted drug delivery to sites within the epidermis and dermis, thereby producing new methods for regulated and contemporary topical use of MTX for psoriasis [105]. Compared with an equivalent ethanol solution, the ethosomes demonstrated superior biocompatibility with human embryonic skin fibroblasts, which implies that the presence of phosphatidylcholine in ethosomal vesicles can enhance biocompatibility. Based on the research, Zhang et al. concluded that ethosomes might improve the trans-dermal delivery of psoralen as well as other drugs that probably require deep skin delivery [106]. Moreover, the increased penetration and deposition of psoralen delivered by ethosomes are advantageous as it can reduce toxicity and enhance the efficacy of psoralen long-term treatment [107]. In addition, as skin vitality decreases, the amount of penetration of psoralen delivered by liposomes is increased, while the deposition of psoralen from liposomes and ethosomes into skin is decreased. This study also showed that there was less deposition of ethosomes- or liposomes-delivered psoralen in the skin, which could relate to decreased cellular uptake [108].
Nanoemulsions
Nanoemulsion is a transparent/translucent, isotropic, heterogeneous system consisting of two immiscible liquids in which drugs are finely dispersed as nanodroplets. An interfacial layer of emulsifier and co-emulsifier are used to stabilize it [109–111]. Somagoni et al. used Vitamin E TPGS through using the solvent evaporation method, and prepared nanoemulsion by the high-pressure homogenization method. They found that nanomiemgel is released in a controlled manner, and induced permeation of aceclofenac and capsaicin that were 2.02 and 1.97-fold higher than Ace-Proxyvon, respectively, via dermatomed human skin. In the rat skin microdialysis study, nanomiemgel also showed that 2.94 and 2.09-fold greater Cmax of aceclofenac and capsaicin, respectively, than Ace Proxyvon [112]. Rajitha et al. found an elevated anti-psoriasis efficacy in low methotrexate nanoemulsion formulations compared with the oral methotrexate formulation [113].
Transfersomes
Cevc first proposed the concept of Transfersomes in 1992. Transfersomes are an ultra-deformable carrier, facilitating the efficient distribution of a wide variety of drugs through the skin barrier [114]. There are two different interdependent mechanisms adopted by transfersomes, which are the use of electromechanics (pressing through intercellular gaps and pores) and the use of the gradient in water activity across the epidermis (dehydration and rehydration cycles) [115]. Eline et al. prepared transfersomes of RNA interferon by solvent evaporation method to certify its effectiveness in the treatment of psoriasis, and found that transfersomes of RNA interferon could effectively improve the pathological conditions of psoriasis [116].
Solid lipid nanoparticle
Solid lipid nanoparticles (SLNs) are an innovative generation of the nanoparticle system composed of a mix of physiological lipids and surfactants [117]. SLNs have unique characteristics including their compact size, high drug loading capacity, large surface area, and prolonged drug release profile consequent to the slow degradation of lipid matrices [118]. The small particle size of SLNs ensures the close contact between the nanocarrier and the stratum cuticle, thus promoting penetration into the skin [119]. Treatment of various skin diseases, including psoriasis, has also utilized SLNs. Sonawane et al. prepared gel formulation that contained calcipotriol and betamethasone dipropionate loaded SLNs (CT-BD-SLNs) to achieve effective treatment of psoriasis [120]. Pradhan et al. established, improved, and tested the Fluocinolone acetonide (FA) combined with SLNs for its anti-psoriatic activity, and they suggested that FA containing SLNs might be a promising carrier for the treatment of psoriasis [121].
Polymer-based nanocarriers
Polymeric nanoparticles
Polymeric nanoparticles (NPs) have excellent biodegradability and biocompatibility that can shield drugs from being degraded and can also envelope both hydrophobic and hydrophilic drugs. Khalid et al. demonstrated that enhancement of bioavailability and long-term retention of the apremilast-loaded PLGA nanoparticles might contribute to once-daily regimen treatment [122]. Tomoda et al. prepared spherical and indomethacin-loaded PLGA nanoparticles through the use of emulsion solvent evaporation method and employed the nanoparticles to transdermal delivery [123]. Shah et al. prepared surface modified bilayered nanoparticles with oleic acid by PLGA and chitosan. Spantide II was added to the inner core of PLG and ketoprofen A to the outer layer of chitosan. Hydroxypropyl methylcellulose (HPMC) and Carbopol were used to produce the nano gel formulation to further boost the delivery of drugs to the deeper skin layers through increasing the contact time with skin and preventing the loss of water [124].
Polymeric micelles
Polymer micelles enhance drug solubility and enhance the distribution of water-soluble drugs and their localization in the skin layer. Polymer micelles have the specific ability to encapsulate hydrophilic drugs. In dermatological diseases such as psoriasis and acne, polymeric micelles could enhance drugs deposition in targeted sites of the skin [125]. Ehrlich et al. investigated the efficacy and safety of paclitaxel in the treatment of severe psoriasis, and found that micellar paclitaxel has a certain therapeutic effect on severe psoriasis [126]. Skin deposition studies showed that the formation of polymer micelle (1.50 ±0.59 µg/cm2) had a higher retention rate than traditional tacrolimus ointment (0.47 ±0.20 µg/cm2), and it was also found that polymeric micelles improved the topical permeability of tacrolimus in psoriasis patients [127].
Polymeric nanocapsules
Polymeric nanocapsules, as a potential drug delivery system, have been extensively studied in recent years. Much research has been carried out to investigate the therapeutic ability of nanocapsules for innocuous and useful delivery of drugs used in different diseases (including psoriasis) [128, 129]. Cationic nanocapsules containing dexamethasone were prepared by Eudragit RS 100 polymers, and the in vitro drug release and skin permeability were assessed. Surprisingly, as a result of their excellent ability to permeate skin to regulate drug release across skin, these nanocapsules have received much attention [130]. Studies on skin permeation using porcine skin indicated that a large number of drugs are present in the viable epidermis – the primary target site for dexamethasone to exert its action against psoriasis. This study implies that suitability of cationic nanocarriers for targeting the epidermis in psoriatic skin as the negatively charged skin surface expedites the penetration of the positively charged particles when applied topically [131]. Beber et al. also produced cationic polymeric nanocapsules containing dexamethasone in a hydrogel formulation. They suggested that nanocapsules are expected to become the transporter for the delivery of dexamethasone [131].
Polymeric nanospheres
Nanospheres are nanocompounds, which are biodegradable, biocompatible, and synthetic polymers-based. Nanospheres (TyroSpheres) have been successfully used to deliver drugs to the skin. Nanospheres provide better solubility with chemicophysical protection of the therapeutic moiety, enhanced absorption and drug release in a controlled manner [132]. Batheja et al. prepared lipophilic drugs that are loaded with tyrosine-derived nanospheres for topical application, and found that the drug permeation of TyroSphere was enhanced compared with aqueous nanosphere formulation. Therefore, TyroSphere is also a useful delivery medium for lipophilic drugs when treating skin conditions such as acne and psoriasis [133]. Another vitamin D3 loaded polymeric nanospheres (TyroSpheres) proposed by Ramezanli et al. also improved drug loading [134]. Jain et al. discussed the antipsoriatic and anti-inflammatory properties of thymoquinone (TMQ), which is an element of the hydroalcoholic extract of Nigella sativa (N. sativa) and is conveyed via nanospheres/lipoglobules (particle size < 70 nm). They supported the potential of TMQ lipospheres for patients with psoriasis [135].
Dendrimers
Dendrimers are monodisperse, multivalent, and typically spherical macromolecules that has a hyperbranched and regular structure, including manageable internal cavities and external border with 3D multifunctional moieties that can be useful for dual drug delivery, as well as increasing the solubility and recognition of drugs [136, 137]. Compared with other polymers, dendrimers have several advantages, such as being less readily absorbed by the reticuloendothelial system, being easy to modify, aiming at specific sites in the body, protecting the reproductive pharmacokinetic behaviour, and supplying different structures to minimize production costs [138]. The dendrimer solution has a unique viscosity generation property that allows a very concentrated formulation to be applied on the skin [139]; dendrimers have been used successfully to deliver anti-psoriatic drugs. Gras et al. investigated whether the second-generation ammonium terminated carbosilane dendrimer (2G-NN16) was a possible agent for the treatment of Th17 deregulated pathologies. They concluded that 2G-NN16-carbosilane dendrimer could be a potential system for the management of IL17-involved autoimmune diseases, such as psoriasis in which IL17 is the main cause of the disease [140].
Conclusions
Psoriasis is a skin disease that is the consequence of a variety of autoimmune cellular processes, including keratinocytes, dendritic cells, T cells, etc. There are various treatments for psoriasis, none of which is completely safe and effective with great patient compliance. So far, existing drugs suppress the condition and ease symptoms but do not completely cure psoriasis, such as methotrexate and cyclosporine. Therefore, a newer drug delivery method has been widely used for antipsoriatic drug delivery, especially nanocarriers. Nanotechnology therapies have improved efficacy and patient compliance without producing adverse events. These methods can enhance the penetration of drug molecules to target sites hence can treat psoriasis of different types. In addition, several nanocarrier-based antipsoriatic agents have been patented, demonstrating the potential functions of such nanocarriers in the treatment of psoriasis. Therefore, nanocarriers is a promising agent to transform the field of clinical dermatology and will eventually become an important treatment for patients with psoriasis.